Background
The introduction of the second-generation TKIs further improved outcomes in BCR::ABL1-positive acute lymphoblastic leukemia (BCR::ABL1+ ALL). Flumatinib is a novel second-generation TKI approved in China in 2019. It has shown better efficacy compared to imatinib in clinical trials of CML, but few reports are available in ALL. Herein, we report the updated results of the RJ-ALL2020.2A trial, which is the first reported registered clinical trial of flumatinib in adult BCR::ABL1+ ALL last year. The enrollment will be completed at the end of Aug. 2023.
Methods
In this prospective phase II study, patient (pt) eligibility mainly includes: 18≤age<65 years; newly diagnosed BCR::ABL+ ALL; with adequate organ function. Once the diagnosis is confirmed, the combination of flumatinib (600mg/day) and VIP-based chemotherapy regimen (Vincristine/Idarubicin/Prednisone) is given promptly. Allo-HSCT is recommended for all eligible pts. Blinatumomab is allowed to administer for MRD clearance before allo-HSCT. All pts receive central nervous system (CNS) prophylaxis once achieving remission. The choice of subsequent salvage treatments is based on the discretion of physicians. Multiparameter flow cytometry (minimal residual disease[MRD]<10 -4) and RT-qPCR (BCR-ABL1 transcript<10 -5) are used to monitor MRD. The primary endpoints are MRD clearance, PFS, and OS. (Clinical Trial Registration Number: ChiCTR2100042248)
Results
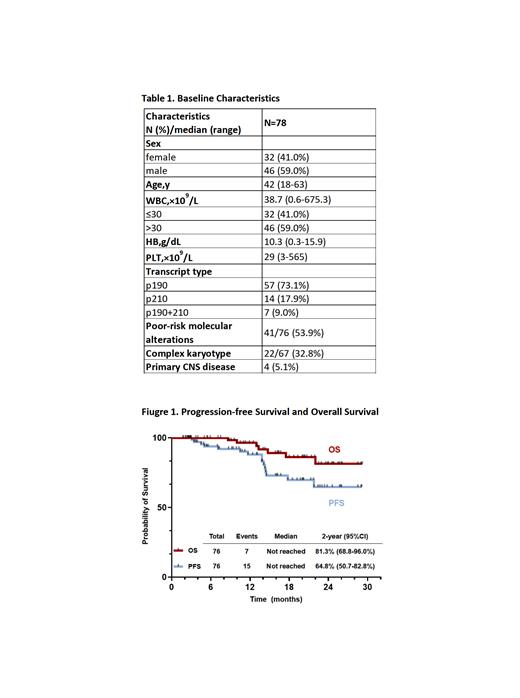
From Dec. 2020 to Jun. 2023, 81 adult pts with de novo BCR::ABL1+ ALL were enrolled. Seventy-eight pts were included in the efficacy and safety assessment (3 were excluded due to the short duration of treatment), and 76 were available for survival analysis (2 withdrew). Median age was 42 years (range, 18-63). Median WBC count at diagnosis was 38.7×10 9/L (0.6-675.3). Four pts had primary CNS leukemia (Table 1).
Among 78 pts, 75 (96.2%) achieved CR/CRi at the end of induction, and 78 (100%) achieved CR/CRi at any time. No early death occurred during the induction course. MRD flow negative rates were 67.1% (51/76) and 79.7% (55/69), while the CMR rates were 31.1% (23/74) and 52.2% (36/69) at the end of induction and 3 months, respectively.
With a median follow-up time of 16.2 months (range, 1.8-29.2), the estimated 2-year PFS and OS rates were 64.8% and 81.3% (Figure 1). Among the 69 survived pts, 61 were in continuous remission, and 8 were relapsed (4 had CNS involvement). Among 7 deaths, 6 died of disease progression (4 had CNS involvement), and 1 of pulmonary infection post-HSCT. There was a survival superiority in pts who achieved MRD flow negativity (PFS: p=0.091, OS: p=0.005) or CMR (PFS: p=0.055; OS: p=0.033) at 3 months. With a median time of 6.4 months (range, 1.6-10.3) from diagnosis, 46 pts (median age of 39 years [range, 20-60]) received allo-HSCT with 45 pts in CR1. The MRD flow negativity and CMR rates were 82.2% (37/45) and 70.5% (31/44) before HSCT, respectively. PFS and OS significantly improved in patients who underwent HSCT (PFS: p<0.001, OS: p=0.012).
In multivariate analysis for PFS and OS, MRD flow negativity at 3 months (PFS: HR: 0.129, p=0.016; OS: HR: 0.151, p=0.029) and allo-HSCT (PFS: HR: 0.247, p=0.036; OS: HR: 0.176, p=0.042) still had a significant effect on PFS and OS as protective factors. When including CMR as a variable, CMR at 3 months (PFS: HR: 0.221, p=0.02; OS: HR: 0.115, p=0.054) and allo-HSCT (PFS: HR: 0.169, p=0.01; OS: HR: 0.139, p=0.021) had the similar impact on survival.
Among 75 pts evaluable for ABL1 mutations, 24 (32%) pts were mutated, and T315I was the most frequently identified (75%). Pts with T315I mutation showed a poorer outcome than non-T315I mutation (PFS: p<0.001, OS: p=0.01).
Meanwhile, the safety profiles of the flumatinib-based therapy were acceptable. Non-hematological AEs were mostly G1-2, which were reversible rapidly after symptomatic management. Most of the G3-4 AEs were hematological, possibly related to chemotherapy. No permanent discontinuation or death related to flumatinib was observed.
Conclusion
This prospective study further confirmed the efficacy and safety of the promising second-generation TKI flumatinib combined with chemotherapy in newly-diagnosed Chinese adult pts with BCR::ABL1+ ALL. Achieving MRD flow negativity or CMR at 3 months and bridging allo-HSCT could further improve survival. The long-term follow-up data will be disclosed soon.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Flumatinib is a novel 2nd-generation TKI used for CML and BCR::ABL1+ ALL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal